

software developments according to ISPE GAMP5 guidelines in the categories 1-5. The platform must, for example, prevent any file destruction or replacement, unless this replacement is traced, dated, and justified. embeX offers software development services according to ISPE GAMP 5. Encryption and data protection to prevent forgery.Access control, with various attributions and rights according to the roles of each individual (trainee, trainer, manager, HR).There are also certain rules on the questionnaire side, with numbered and blocked multiple choice questions.
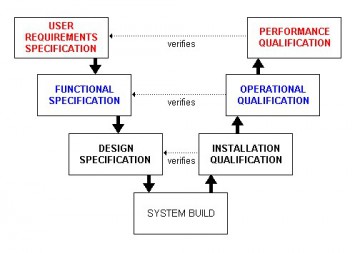

At each step, an electronic signature is required to guarantee the authenticity of the user. Each employee must have his/her training file, with training courses followed in detail (a simple YES / NO is not enough), and diplomas and certificates attesting to skill acquisition.This document must be reliable, authentic, dated and signed. Each training course must be documented on paper, with a list of employees who completed the course and obtained a diploma or certification.Precisely, these are the rules to be followed, and which Dokeos LMS includes by design: GAMP5 is a set of guidelines for manufacturers and users of automated. In short, that it is in line with its training obligations, without possible doubt nor falsification. FDA has proposed a new guideline Computer Software Assurance for. Recommendations on how the instrument software can be implemented for compliance with 21 CFR Part. The purpose of an LMS in case of an FDA or EMA audit is to show that the laboratory, dispatcher or supplier has trained the right people, at the right time, in the right procedures. This version is regarded as the most structured and project based approach and is more inclined in ensuring risk control and quality management of. Generally, GAMP5 refers to a guidance document entitled GAMP5: A Risk-Based Approach to Compliant. GAMP-5 or version 5 of GAMP is the latest standard of the guidelines and was released in February 2008 by the International Society for Pharmaceutical Engineering (ISPE) a GAMP partner company. Dokeos LMS, in line with GAMP 5 guidelines GAMP stands for Good Automated Manufacturing Practice.


 0 kommentar(er)
0 kommentar(er)
